HistDAO™ 60 Tablets (new formula)
Clinical Applications
» Supports Healthy Degradation of Food-Derived Histamine*
» Enhances the Presence of Diamine Oxidase in the Digestive Tract*
$58.99
Description
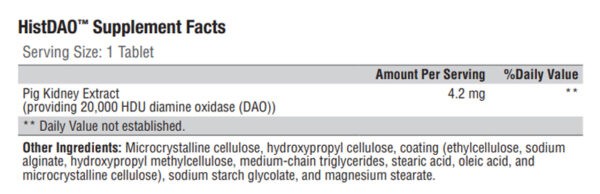
HistDAO™ provides a concentrated source of diamine oxidase (DAO)—the main enzyme responsible for the degradation of ingested histamine. This formula delivers the clinically researched dose of DAO in an easy-to-swallow, gastro-resistant mini-tablet, which is essential for releasing DAO in the small intestine, where it breaks down food-derived histamine.*
Discussion
Histamine is a bioactive amine-signaling molecule generated in the body in response to allergens, injuries, foreign substances, and alcohol. It has an array of physiological effects, including increased blood supply to sites in the body that cause reactions such as itching and flushing of the skin, low or high blood pressure, rapid heartbeat, headache, and other symptoms typically experienced in individuals with allergies.1,2 Apart from its role in the inflammatory response, histamine can have varied systemic effects ranging from increased stomach acid production, blood vessel permeability, contraction of smooth muscle tissues, and immune cell modulation. The highest histamine concentrations in the body are found in the gastrointestinal (GI) tract, lungs, and skin, with lesser amounts in the brain and heart.1-3 Two enzymes work within the body to counteract the effects of excess histamine. Histamine-N-methyltransferase works throughout the body, including in the brain, to break down histamine created intracellularly, and the digestive enzyme diamine oxidase (DAO) breaks down exogenous histamine that enters the body through food consumption.1,4*
Histamine is present in various concentrations in many foods, but most notably in fermented foods, such as sauerkraut, sausage, cheese, yogurt, and alcoholic beverages. Certain drugs and non-histamine–containing foods can also play a role by promoting histamine release or inhibiting DAO.1,5 Primarily active in the small intestine, DAO degrades histamine to the inactive metabolite imidazole acetaldehyde to control the histamine burden from food, supporting a steady histamine balance.1,5,6 In individuals lacking in DAO, a disproportionate accumulation of plasma histamine can lead to a range of adverse symptoms and may result in histamine intolerance (HIT)—a type of food intolerance resulting from excess histamine ingestion.2 A person’s histamine tolerance varies according to their threshold, which is how much histamine their body can tolerate before experiencing symptoms. Detailed questionnaires, food
intake logs, trials with a low-histamine diet, and measurements of DAO and histamine can help assess HIT.2,5,7*
An estimated 1% of the population has HIT, and most are middle-aged or older.1 It has been suggested that individuals with DAO deficiency are often unaware of its existence, meaning that it could be more widespread than known. Food intolerances, such as HIT, can be much more difficult to pinpoint than food allergies; when an individual has HIT, the reaction is typically dependent on the build-up of histamine and an individual’s histamine threshold, whereas with food allergies, an immune response occurs almost immediately after ingesting the allergenic food.1 Additionally, genetic and environmental factors influence DAO metabolism and histamine tolerance. Individuals with GI conditions, those with inflammatory skin and respiratory conditions, and those taking certain medications may be at higher risk for altered DAO production.1-3*
Supplementation with DAO has been well-studied and appears to have a viable role in supporting individuals who may be deficient in this histamine-degrading enzyme.2,5 Headaches are one of the most frequently reported symptoms related to HIT. In a double-blind, randomized trial, subjects (N = 100) who regularly experienced episodic migraines and were found to have a DAO deficiency assessed through plasma testing were given supplements containing 20,000 histamine digesting units (HDU) of DAO per dose for 1 month. Compared with placebo, subjects receiving the DAO supplements exhibited a significantly reduced duration of their migraine attacks.3
*
HistDAO™ contains a multi-patented, gastro-resistant source of diamine oxidase (DAO) provided at the clinically researched dose of 20,000 HDU in an easy-to-swallow mini-tablet. This formula was designed to deliver an exogenous source of DAO to the small intestine, where it supports the degradation of dietary histamines. HistDAO™ is not a formula for use by individuals with diagnosed immune-related food allergies (eg, peanuts, shellfish, etc.).*
DIRECTIONS: Take one tablet no more than 15 minutes before the consumption of histamine-rich foods, or take as directed by your healthcare professional.
Consult your healthcare professional prior to use. Individuals taking medication should discuss potential interactions with their healthcare professional. Avoid if allergic to pork or any other ingredient. HistDAO™ is NOT EFFECTIVE for symptoms of immune-related food allergies, such as peanuts, shellfish, etc., or for gluten intolerance due to sensitivity or celiac disease.
STORAGE: Keep closed in a cool, dry place out of reach of children.
FORMULATED TO EXCLUDE: Wheat, gluten, corn, yeast, soy, dairy products, fish, shellfish, peanuts, tree nuts, egg, sesame, ingredients derived from genetically modified organisms (GMOs), artificial colors, and artificial sweeteners.
References
1. Maintz L, Novak N. Am J Clin Nutr. 2007;85:1185-1196. doi:10.1093/ajcn/85.5.1185
2. Schnedl WJ, Enko D. Nutrients. 2021;13(4):1262. doi:10.3390/nu13041262
3. Izquierdo-Casas J, Comas-Basté O, Latorre-Moratalla ML, et al. Clin Nutr.
2019;38(1):152-158. doi:10.1016/j.clnu.2018.01.013
4. Yoshikawa T, Nakamura T, Yanai K. Int J Mol Sci. 2019;20(3):737.
doi:10.3390/ijms20030737
5. Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, et al. Biomolecules.
2020;10(8):1181. doi:10.3390/biom10081181
6. Yacoub MR, Ramirez GA, Berti A, et al. Int Arch Allergy Immunol. 2018;176(3-4):268-
271. doi:10.1159/000488142
7. Komericki P, Klein G, Reider N, et al. Wien Klin Wochenschr. 2011;123(1-2):15-20.
doi:10.1007/s00508-010-1506-y
8. Schnedl WJ, Schenk M, Lackner S, et al. Food Sci Biotechnol. 2019;28(6):1779-1784.
doi:10.1007/s10068-019-00627-3
All XYMOGEN® Formulas Meet or Exceed cGMP Quality Standards.
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease